
At each of these three stages there are factors that
modify the effects and outcomes of exposure. In the
absence of specific knowledge, the most common
preventive strategy is to reduce supersaturation by
limiting the depth/time exposure and ascent rate for
all divers to avoid substantial bubbling — even though
bubbles put only some divers (those with a right-to-
left shunt [RLS]) at risk. Without bubbles (or with
only a few), there will be no arterialization, even in
divers with RLS, and there will be no DCS in those
susceptible to arterial embolization.
Several approaches to mitigating postdive bubble
occurrence have been studied; these include predive
removal of hypothetical bubble nuclei by whole-
body vibration, attempts to influence oxygen radicals
or nitric oxide suspected of contributing to bubble
generation, stimulation of heat-shock protein
production and various other methods, including
predive chocolate treats. Although some of these
factors may reduce the amount of bubbles, the effects
vary and may be of less importance than the individual
variation in response to decompression.
If every diver had a consistent individual propensity
for VGE production and could be classified as a
“bubbler” or “nonbubbler” across a broad spectrum of
reasonable dive exposures, safe exposure limits could be
tailored individually. We would achieve greater precision
if we could identify those divers who have persistent
or occasional RLS and then customize a dive exposure
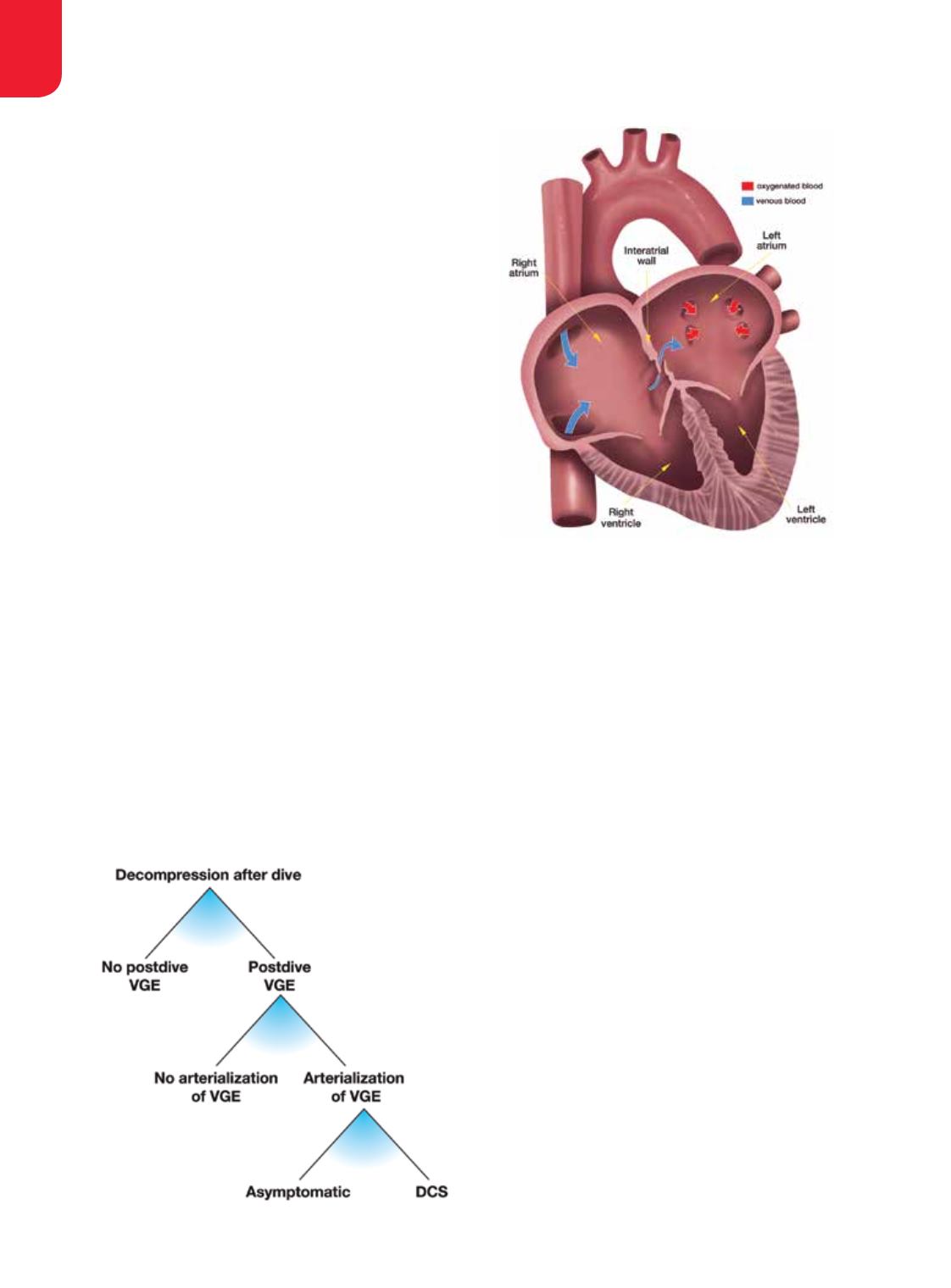
for them. At present we already know that a large
patent foramen ovale (PFO) enables arterialization and
apparently increases the risk of DCS. A test for PFO is
available. We can identify divers with a large PFO and
close it, but this does not solve the problem for all divers
because RLS may occur in lungs regardless of PFO. Risk
of DCS due to pulmonary RLS may be more difficult to
tackle because pulmonary RLS seems to be part of the
normal physiological response to exercise and far more
prevalent in the population than large PFOs.
It is important to note that even if we could prevent
the occurrence of VGE, DCS could still occur. VGE
do not play a role in pain-only DCS. Some cases of
spinal DCS may be caused by bubbles occurring
locally in tissue, without bubbles in venous blood.
Similarly, some cases of inner-ear DCS may be caused
by local bubbles rather than by arterialized gas emboli.
Cutaneous (skin) manifestations of DCS may be caused
by various mechanisms, some involving embolization
of arterialized VGE and others independent of VGE.
As in lung cancer, the definitive cause of DCS is
an external physical factor that, unlike smoking, we
cannot eliminate if we want to dive. But by controlling
the magnitude of exposure, we can minimize the risk
of DCS. There is still a lot of room to improve the
precision of DCS prediction and develop individually
tailored preventive exposure restrictions. This can be
achieved by advancing VGE study methods and dive
population studies to identify individuals who may
easily produce VGE or are prone to arterialization.
Understanding why some divers easily produce VGE
and why some are more prone to DCS in the case of
RESEARCH, EDUCATION & MEDICINE
EXPERT OPINIONS
52
|
WINTER 2016
Figure 1.
















